Cores
- Transgenic and KO Mice
- Metabolic & Molecular Physiology Core
- Genomics & Epigenetics Core
- Human Genetics Core
Services Provided by the Metabolic & Molecular Physiology Core:
In vivo Indirect Calorimetry and Body Composition. Indirect calorimetry to assess oxygen consumption/caloric expenditure and substrate utilization, as well as movement patterns and food and water consumption during light and dark cycles is performed on mice using Columbus Instruments Oxymax metabolic chambers. Animals are acclimated for 24 h prior to a 48 h testing period (25-26). Body composition can be assessed either by DEXA (Lunar PIXImus Densitometer, GE Medical Systems) or MRI (EchoMRI 3 in 1 Body Composition Analyzer) (25-27). The MRI instrument can also estimate tissue total lipid composition ex vivo for investigators who require immediate and inexpensive analyses in an easily excised organ or tissue bed(25).
In vivo Insulin Sensitivity. Insulin tolerance: IP-ITT or IV-ITT with 2-deoxyglucose. The insulin tolerance test is a non-terminal test to assess whole body insulin sensitivity. To determine the rate and site of glucose disposal, [3H]-2-deoxy-d-glucose (10Ci/mouse) is infused IV (jugular cannula) into the mouse in combination with insulin. Tissue glucose uptake is determined by the rate of disappearance of 2-[3H]-deoxyglucose from the circulation along with counts of phosphorylated 2-deoxy-D- [2-3H] glucose in individual tissues. Tissues are harvested for post-insulin signal transduction analyses by immunoblotting. Pyruvate tolerance tests to assess gluconeogenic capacity are performed upon request. Additionally, radiolabeled glucose uptake assays can be conducted under thermal challenge to assess substrate demand in rodent tissues including brown adipose.
Euglycemic-hyperinsulinemic clamp. The euglycemic-hyperinsulinemic clamp technique is the gold standard method for quantification of in vivo insulin sensitivity. Radiolabeled glucose is used to determine tissue insulin sensitivity (muscle, liver, adipose tissue) between genotypes of mice under various dietary conditions. Mice are chronically catheterized using dual lumen cannulas surgically placed into the right jugular vein (25-26, 28-29). Seventy-two hours after surgery glucose turnover is measured at basal and during hyperinsulinemia. The glucose infusion rate (GIR) is determined by the amount of exogenous dextrose necessary to maintain euglycemia. The insulin-stimulated glucose disposal (IS-GDR) and hepatic glucose production (HGP) rates are determined using the Steele equation (30). All individual raw data as well as mean ± SEMs for basal and clamp glucose concentration, clamp circulating plasma and infusate insulin concentrations, basal glucose turnover rate, as well as steady state GIR, IS-GDR, and HGP will be provided. Tissues are harvested post-clamp and insulin signal transduction is assessed by immunoblotting as we have described previously (25-26, 28-29).
Ex vivo tissue metabolism and insulin action. To quantify insulin action in skeletal muscle, extensor digitorum longus (EDL), tibialis anterior (TA), and soleus (SOL) muscles are harvested from mice (4-6 h fasted condition) and incubated in 2-deoxyglucose in the presence (stimulated) or absence (basal) of physiological insulin and [3H]-2-deoxy-d-glucose (25, 31). Small molecule compounds and hormones (e.g. AICAR, Metformin, SIRT modulators, estradiol, isoproterenol) can be added to the incubation medium to challenge metabolism in this controlled setting. A similar approach can be performed on white and brown adipose tissue sections.
Cellular/Molecular Metabolism and Insulin Action. Skeletal muscle satellite cells are isolated from genetically engineered mice or from human muscle biopsies and differentiated to myotubes. Primary cells can also be obtained from adipose tissue beds (white or brown) and liver. Assays to investigate substrate metabolism and insulin action (insulin-stimulated 2-deoxyglucose uptake and insulin signal transduction) are performed on primary cells under specified conditions (25, 29). In addition, primary cells can be transferred to the Mitochondrial Biology Sub-core for assessment of oxygen consumption by Seahorse Biosciences technology. These techniques to assess insulin action and metabolism are also routinely performed on standard cell lines genetically altered by viral-mediated knockdown approaches. Cellular crosstalk assays to determine whether secreted factors can induce altered metabolism or insulin action of a second cell type can be performed by special request (25, 29). Furthermore, conditioned-media from primary cells can be harvested and transferred to the Inflammatory Signaling Sub-Cores or Core E - TPAC for factor/lipidomics identification by mass spectroscopy analysis.
In vivo Oxidative Metabolism and ROS Assessment. Indirect calorimtery assessment by CLAMS including oxugen consumption, respiratory exchange ratio, ambulatory movement, and feeding. ROS can be assessed in tissues by fluorescent labeling and posttreatment quantitation by fluorescence imaging.
Ex vivo Oxidative Metabolism and ROS assessment. Substrate oxidation and ROS assays are performed on small tissue explants and isolated muscle (EDL, soleus or TA) using newly acquired high-resolution respirometry (Oroboros), or in primary myocytes or mitochondrial suspensions prepared according to the methods of (32) with modifications described by Koves et al. (33) and Kim et al. (34). Oxidation rates are determined by radioactive 14CO2 counts assessed by liquid scintillation counting and substrate deposition (esterification and glycogenesis) determined by tracer incorporation into the storage pool (13, 25).
Exercise Capacity and Performance. The MMPC has recently acquired 36 mouse running wheels, a rodent treadmill, and instrumentation to test muscle strength and muscular endurance (active hanging and ladder climbing). Dr. Hevener and her team have extensive experience in the exercise field and are able to deliver comprehensive data sets on metabolic responses to acute and chronic exercise.
Tissue/cell fixing and sample delivery to either UCSD or UCLA Histopathology Shared Resource or the UCLA, UCSD EM Core, UCLA Brain Research Institute or UCLA Mitochondrial Biology Core can also be performed by the MMPC. Reduced fees for microscopy core training and services performed by the UCLA-BRI have been negotiated for all UCSD-UCLA DRC members. Furthermore, Dr. Hevener has obtained numerous tools to assess additional aspects of mitochondrial function and turnover/mitophagy, and these procedures and reagents can be made available upon request.
 The Extracellular Flux Analyzer (3xXF-96 and 3xXF-24 Seahorse Biosciences/Agilent Technologies) allows for measurements of oxygen consumption rates (OCR) and extracellular acidificiation rates (ECAR) in a 24 or 96-well plate format in intact cells, permeabilized cells, spheroids, and isolated mitochondria. Intact cell respirometry - the most commonly used respirometry assay is completed in intact cell lines and primary cells. This assay provides first pass information on the changes in mitochondrial function in response to compound treatment, nutrient modifications, or genetic alterations. Isolated mitochondria respirometry - provides more mechanistic insight into the mitochondrial phenotype in response to treatments. Mitochondria are isolated from animal tissues or cell lines immediately before running the respirometry assay. Spheroids or permeabilized cell respirometry in permeabilized cells allows for a more mechanistic approach while maintaining cellular architecture. This assay will be used for more in-depth studies and requires permeabilization reagent exclusively offered through Seahorse Biosciences. Spheroid respiration requires a specialized plate that is available to run assays on 3D cell structures (e.g., pancreatic islets), in order to maintain a more physiological cellular environment.
The Extracellular Flux Analyzer (3xXF-96 and 3xXF-24 Seahorse Biosciences/Agilent Technologies) allows for measurements of oxygen consumption rates (OCR) and extracellular acidificiation rates (ECAR) in a 24 or 96-well plate format in intact cells, permeabilized cells, spheroids, and isolated mitochondria. Intact cell respirometry - the most commonly used respirometry assay is completed in intact cell lines and primary cells. This assay provides first pass information on the changes in mitochondrial function in response to compound treatment, nutrient modifications, or genetic alterations. Isolated mitochondria respirometry - provides more mechanistic insight into the mitochondrial phenotype in response to treatments. Mitochondria are isolated from animal tissues or cell lines immediately before running the respirometry assay. Spheroids or permeabilized cell respirometry in permeabilized cells allows for a more mechanistic approach while maintaining cellular architecture. This assay will be used for more in-depth studies and requires permeabilization reagent exclusively offered through Seahorse Biosciences. Spheroid respiration requires a specialized plate that is available to run assays on 3D cell structures (e.g., pancreatic islets), in order to maintain a more physiological cellular environment.
 Cellular Imaging. The Operetta allows for high content screening, with confocal capabilities, of mitochondrial physiology. The ability to use the Operetta to increase throughput decreases the time and effort involved with running these assays compared to more traditional imaging systems. Additionally, since the Operetta fits on the bench-top, it can be used in the same location as other Core instruments, thus, increasing efficiency. The Operetta also offers specialized Harmony imaging software for improved data analysis.
Cellular Imaging. The Operetta allows for high content screening, with confocal capabilities, of mitochondrial physiology. The ability to use the Operetta to increase throughput decreases the time and effort involved with running these assays compared to more traditional imaging systems. Additionally, since the Operetta fits on the bench-top, it can be used in the same location as other Core instruments, thus, increasing efficiency. The Operetta also offers specialized Harmony imaging software for improved data analysis.
Biochemical Assays. ATP synthesis measurements can be performed using a Bioluminescence assay run on isolated mitochondria in a plate reader. Total cellular ATP and other high energy substrates can also be measured following cell lysis. The MMPC can also coordinate additional metabolite analyses by providing samples to the UCLA Metabolomics Shared Resource.
 Luminex and MSD MesoScale Multiplexing assays.
Luminex and MSD MesoScale Multiplexing assays. The Inflammatory Signaling sub-core offers a broad range of reliable and sensitive assays to meet the needs of the diverse DRC membership. In order to provide the most value to MMPC users, sample analyses are performed with either MSD MesoScale or Luminex microsphere-based multiplex assays, using standardized quality-controlled biomarkers. The MSD MesoScale QuickPlex instrument offers an expanded broad range, highly sensitive, low sample volume assessment of analytes in biological samples including plasma, tissues, culture media, and cell lysates. This instrument can replace standard ELISA and immunoblotting assays for assessing circulating factors (e.g. hormones, chemokines, and cytokines) and cell or tissue proteins/protein signaling events. Additionally, MSD offers assay development reagents and plates suitable for immunogenicity, PK (pharmacokinetics), serology, and cell binding. MSD provides an open technology platform that allows researchers to develop novel singleplex or multiplex assays quickly using their own in-house or commercially available capture/detection antibody pairs. This is particularly useful for researchers wanting to develop customized multiplex assays or wishing to convert ELISAs or other binding assays to the MSD platform. MSD uses MULTI-ARRAY technology combined with electrochemiluminescence to bring speed and high density of information to biological assays. In combination with MULTI-SPOT plates, this technology enables precise quantitation of multiple analytes in a single sample requiring less time and effort than other assay platforms. Luminex 100 xMAP technology, uses color-coded microsphere sets conjugated with specific antibodies or oligonucleotides to permit the capture and detection of specific analytes from a sample. Microspheres are currently available in 100 different colors, each of which can be used to perform a separate assay. Multiplex assays are performed in 96 well microplates, where samples are incubated with a mixture of analyte-specific, color-coded microspheres, and fluorescently-tagged analyte-specific probes. Samples are analyzed to determine microsphere-bound fluorescent intensity values for each analyte. The Luminex 100 instrument robotically aspirates each sample, segregating the microsphere mixtures into individual particles as they pass before two detectors: a red laser excites the microsphere-specific dye signal to identify the type of microsphere particle and a green laser excites fluorescently-labeled specific antibody captured during the assay. Multiplex analyses require reduced sample volume as multiple assays can be performed within in single well, and the sensitivity and dynamic range of the laser-excited fluorescent-dye assay is superior to conventional assays. Multiplex assays offered through the MMPC, and their rationale for inclusion, are listed below and may be customized by request to include any combination of analytes from a given assay panel. Core users may also request custom assay panels that combine components of different assay panels, providing the assay conditions for the merged components are compatible. Multiplex samples are analyzed in duplicate, and most assays require 10-25 l of serum, plasma or cell culture supernatant per sample well.
Atherosclerotic Lesion Analyses. Atherosclerosis is strongly linked to diabetes and commonly co-exists with metabolic dysfunction in susceptible animal models. Cardiovascular complications are an important cause of morbidity and mortality in patients suffering from insulin resistance and diabetes. Sub-core D will focus on cardiovascular complications of diabetes offering expertise to evaluate cardiovascular function in mice by providing the following services: Computerized non-invasive blood pressure measurements, quantification of the extent and severity of atherosclerosis by en face atherosclerotic lesion measurement (25, 39-42), lesion composition and complexity (43), measurement of cardiac function and fibrosis (44), and monocyte/macrophage isolation and phenotyping (25). Aortic root atherosclerotic lesion analysis is performed on fixed heart sections beginning at the aortic valve. Sections are cut until the aortic valve leaflets are visible. Sections are stained with Oil Red O, or can be immunostained for a specific target protein upon investigator request. The slides are examined using a Nikon Eclipse 80i microscope and captured images (magnification 400X) are analyzed using Image Pro Plus 5.1 software (Media Cybernetics, Silver Spring, MD). Lesion complexity score can also be provided using the method of Park et al. (45). This involves counting the lesions containing necrotic lipid cores, cholesterol clefts or fibrous caps and dividing this number by the total number of lesions in the same section. The data are expressed as percentage of lesions exhibiting complexity. The MMPC has personnel trained in necropsy and tissue preparation for both light and electron microscopy. Morphological and histological analyses can be performed using stereomicroscopy, bright field, phase contrast and fluorescence microscopy, and the MMPC can quantify the various cellular or histological components of the images.
Myeloid cell activation is shown to impact adiposity, insulin action and atherosclerotic lesion development. The DRC Executive Committee decided that the Inflammation Core-Sub Core should continue to provide valuable human/mouse monocyte/macrophage isolation and phenotyping services upon special request. Blood is collected in Vacutainer CPT tubes incubated with RosetteSep monocyte enrichment cocktail to crosslink granulocytes, T-, B- and NK-cells to red blood cells (RBCs). RBCs and negatively-selected cells crosslinked to RBCs are precipitated through the density medium by RT centrifugation. Monocyte-enriched samples are historically 874% CD45+CD14+ monocytes, and typically yield 300 ng/ml of the starting whole blood volume. Bone marrow and adipose tissue macrophages are isolated as previously described (25, 29). Cell pellets can either be frozen at -80 C for subsequent protein or RNA analysis by the investigator or DRC Genomics and Epigenetics Core or delivered to the non-DRC supported FACS Core for additional phenotyping.
Note: By special request, the MMPC is able to transfer biological samples between DRC cores and or institutional cores.
Figure 1. UCSD-UCLA DRC Leadership and MMPC Organizational Structure
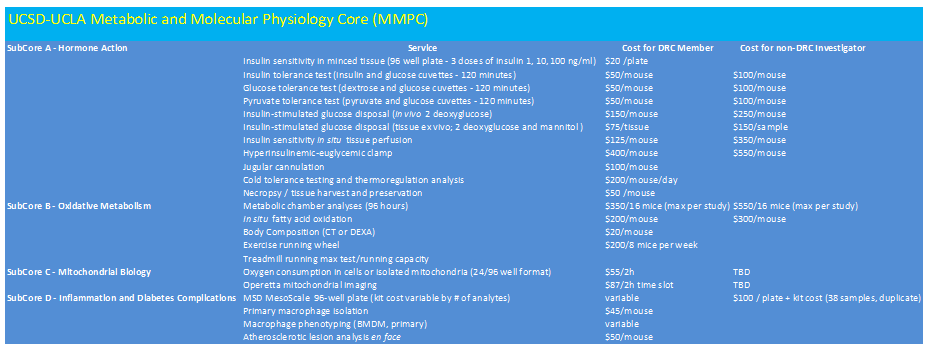
Table 2. MMPC Services and Fee Structure
Andrea Hevener, PhD
Core Director
UCLA Department of Medicine
Division of Endocrinology, Diabetes, & Hypertension
650 Charles E. Young Drive South
CHS Suite 34-115
310-267-5884
ahevener@mednet.ucla.edu
Orian Shirihai, MD, PhD
Core Co-Director
UCLA Department of Medicine
Division of Endocrinology, Diabetes, & Hypertension
650 Charles E. Young Drive South
CHS South Tower
Suite 27-200
310-825-8630
oshirihai@mednet.ucla.edu
Linsey Stiles, PhD
Research Associate
UCLA Department of Medicine
Division of Endocrinology, Diabetes, & Hypertension
650 Charles E. Young Drive South
CHS South Tower
Suite 27-200
310-825-8630
lstiles@mednet.ucla.edu